Patient safety in medical research is paramount, ensuring that individuals who volunteer for studies are protected from harm and treated ethically. The importance of Institutional Review Boards (IRBs) cannot be overstated, as they play a crucial role in overseeing clinical research ethics and safeguarding the rights of participants. With recent funding cuts disrupting vital research programs, like those at Harvard, the comprehensive oversight necessary for maintaining patient safety is jeopardized. The ramifications of halted studies extend beyond just the immediate impact on research; they can diminish public trust in medical research and hinder advancements that benefit all of society. As we navigate these challenges, it is essential to reaffirm our commitment to patient safety and the ethical frameworks that govern the medical research landscape.
The protection of individuals participating in clinical trials is a critical aspect of scientific inquiry in healthcare. This concept encompasses various terms, such as the safeguarding of research subjects and the adherence to ethical standards in human experimentation. The role of regulatory bodies, like the IRBs mentioned earlier, is to ensure that all clinical investigations comply with necessary laws and ethical norms to uphold participant welfare. Due to recent financial constraints impacting research funding, the ability to effectively monitor and oversee these studies faces significant threats. As we explore this topic, we must recognize the linked dimensions of research integrity and community trust that are essential to the progress of medical science.
The Importance of Institutional Review Boards (IRBs) in Medical Research
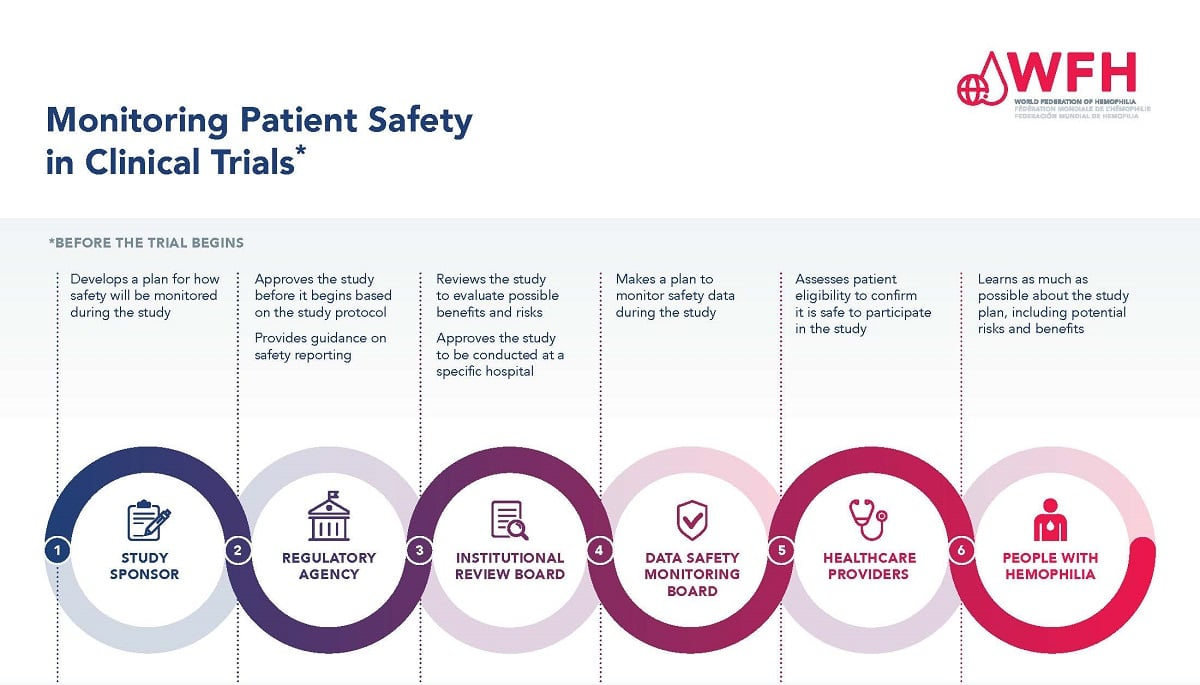
Institutional Review Boards (IRBs) are critical in safeguarding the rights and welfare of participants in medical research. They meticulously review research proposals to ensure that ethical standards are maintained throughout the study process. By doing so, IRBs help ensure that researchers address the risks involved, consent processes, and the overall study design before participants are recruited. This oversight is especially important in contexts involving vulnerable populations, where the potential for coercion or exploitation may be present. As the backbone of clinical research ethics, IRBs vet every detail of the research plan, ensuring that the benefits outweigh the potential harms, thereby upholding public trust in the research community.
Moreover, IRBs act as a point of education for researchers about their ethical obligations. They not only review but also guide investigators on best practices for participant recruitment, retention, and informed consent. Their role extends beyond approval; they provide assurances to participants that ethical considerations are paramount. This systematic oversight prevents unethical practices and promotes transparency, which are increasingly vital in an era where funding cuts threaten the integrity and scope of medical research.
How Funding Cuts Hindering Patient Safety in Medical Research
The recent funding cuts, particularly the freeze on federal research grants, pose a substantial threat to patient safety in medical research. With programs like SMART IRB facing stop-work orders, ongoing studies are already experiencing interruptions that prevent the addition of new clinical sites. This disruption can lead to delays in critical medical advancements and affect the comprehensive oversight necessary to ensure participant safety. As researchers are left scrambling for alternative funding sources, the reduction in resources directly impacts the research infrastructure that guarantees participant rights and welfare.
Furthermore, funding interruptions can result in a ripple effect, slowing down the speed of scientific progress and potentially jeopardizing clinical trials that are crucial for the development of new treatments. As studies are halted or delayed, not only are the participants left in limbo regarding their health but also the research community faces a growing distrust from the public. The pervasive concern is that without adequate funding and support, the ethical oversight necessary to safeguard participants may falter, increasing the risk of exploiting vulnerable individuals who volunteer for clinical studies.
The Intersection of Ethics and Funding in Medical Research
Ethical conduct in medical research is heavily intertwined with funding. As financial resources dwindle due to funding cuts, the ability of institutions to uphold ethical standards diminishes. This is particularly crucial in areas where collaborative research is necessary, as reduced funds can lead to insufficient oversight by IRBs. This raises pressing concerns about research ethics — when budgets are tight, some institutions might prioritize research output over participant safety, leading to potentially harmful consequences.
Conversely, robust funding facilitates the establishment of rigorous oversight mechanisms, allowing IRBs to function effectively. This enables thorough examination of study protocols and participant interactions, fostering an environment where ethics can flourish alongside scientific inquiry. It is imperative for stakeholders in the medical community, including funding agencies and research institutions, to recognize the intrinsic link between financial support and ethical research practices to ensure the continuous protection of those involved.
The Role of IRBs in Collaborating Research Efforts
IRBs play a vital role in facilitating collaborative research across multiple sites, which is essential in addressing complex health issues. For instance, with diseases such as Alzheimer’s requiring diverse expertise from various institutions, IRBs help streamline the review process through systems like the SMART IRB. This single IRB approach minimizes redundancies, enabling institutions to work together efficiently while maintaining adherence to ethical standards. Such collaboration accelerates the pace of medical research by allowing studies to expand quickly and effectively without compromising participant safety.
However, funding cuts threaten this collaborative spirit by curtailing resources necessary for IRBs to function optimally. When financial support is limited, IRBs may lack the personnel or technological resources needed to manage multiple-site studies effectively. This could lead to delays in approvals, decreased ethical scrutiny, and a heightened risk of oversight deficiencies. Ultimately, it underscores the need for sustained funding to bolster collaborative efforts and ensure patient protection remains at the forefront of medical research.
Historical Context of Ethical Oversight in Medical Research
The historical context of medical research is rife with examples that highlight the importance of ethical oversight. Events such as the Tuskegee Syphilis Study and the Willowbrook Hepatitis Study serve as stark reminders of what can go wrong when ethical considerations are disregarded. These incidents led to a significant loss of public trust and necessitated the establishment of comprehensive regulations governing medical research, including the formation of IRBs. By examining these historical injustices, we can appreciate the critical role that IRBs and ethics boards play in preventing recurrence and ensuring that modern research upholds the highest ethical standards.
Current regulations and oversight practices are rooted in the lessons learned from these past atrocities. The establishment of stringent approval processes and informed consent protocols is designed to protect human subjects from exploitation. However, as funding cuts threaten the operational capacity of IRBs, the risk remains that the ethical framework built over decades could start to erode. Continual investment in research ethics is critical to safeguard against historical missteps and to maintain the public’s confidence in the research process.
Consequences of Disrupted Oversight in Multi-site Research
Disruptions in oversight due to funding cuts can have detrimental effects on multi-site medical research. The intricate web of collaborations that characterizes modern research relies heavily on robust oversight by IRBs to ensure that all sites adhere to ethical standards. When funding is halted, it complicates the ability to effectively monitor and manage these collaborations, leading to potential gaps in ethical compliance. This can jeopardize participant safety and diminish the quality of the research outcomes.
The immediate consequence of halted oversight can be seen in the form of delayed patient recruitment, increased risk of ethical breaches, and a decrease in participant confidence. Participants may question whether their involvement in a study is being adequately monitored, possibly dissuading them from volunteering. This erosion of trust can have long-term implications for the research community, as it can lead to ongoing recruitment challenges and reduced public willingness to participate in future studies.
Patient Safety and the Future of Medical Research
The focal point of any medical research endeavor must be patient safety. As funding cuts continue to threaten the operational capacity of oversight bodies like IRBs, the implications for patient safety become increasingly concerning. Innovations in medical treatments, which often hinge on successful clinical trials, depend on meticulous oversight to mitigate risks to participants. When funding sources dwindle, researchers may be compelled to cut corners, potentially jeopardizing patient welfare in the process.
As we move forward, it is essential to emphasize that sustainable funding is not just a financial issue but a moral imperative. Investing in the structures that uphold patient safety ensures that advancements in medical research do not come at the expense of those who bravely volunteer to participate. The interplay between funding and patient safety is crucial; without a commitment to both, the integrity of medical research and the welfare of participants could be severely compromised.
The Need for Innovations in Research Funding
Innovations in research funding are necessary to adapt to the changing landscape of medical research. With frequent cuts to federal funding programs, alternative funding mechanisms, such as public-private partnerships or crowdfunding initiatives, are becoming increasingly relevant. These novel funding approaches can help diversify the financial support for medical research, ensuring that ethical oversight can persist even in financially challenging times. Additionally, creative funding solutions can also engage community stakeholders, thereby enhancing transparency and public trust in the research process.
Moreover, exploring additional avenues for funding can enable institutions to maintain robust IRB programs. This is crucial for upholding ethical research practices and participant safety. As researchers seek out new partnerships and innovative funding sources, it will be imperative that ethics remain a central consideration, ensuring that money does not overshadow the fundamental principles of patient care and safety.
Frequently Asked Questions
What is the importance of IRB in ensuring patient safety in medical research?
Institutional Review Boards (IRBs) play a critical role in ensuring patient safety in medical research by meticulously reviewing research proposals. They are responsible for safeguarding the rights and welfare of research participants by evaluating aspects such as informed consent processes, risk assessments, and overall research integrity. This regulatory oversight is essential for upholding ethical standards in clinical research.
How do funding cuts affect patient safety in medical research?
Cuts in funding can severely disrupt patient safety in medical research. Such financial constraints can halt ongoing studies, limit the ability to launch new clinical sites, and undermine the oversight mechanisms provided by IRBs. This could lead to increased risks for participants and erode public trust in research practices, ultimately compromising the safety and rights of those involved in medical studies.
How does medical research oversight protect patients?
Medical research oversight, particularly through the work of IRBs, protects patients by ensuring rigorous evaluation of research proposals before they proceed. This includes assessing potential risks, ensuring informed consent, and monitoring ongoing studies for compliance with ethical standards. By maintaining a system of checks and balances, oversight helps to minimize harm and uphold the safety of participants in medical research.
What impact do IRBs have on clinical research ethics regarding patient safety?
IRBs significantly influence clinical research ethics by enforcing guidelines that prioritize patient safety. They assess the ethical implications of research studies, evaluate the potential benefits against risks, and ensure that participant consent is obtained properly. By doing so, IRBs not only protect individual participants but also enhance the integrity and credibility of the broader research field.
Why is patient safety critical in medical research, especially in studies like the Harvard medical study?
Patient safety is paramount in medical research, including studies like those conducted at Harvard, as it directly affects the well-being and rights of participants. Ensuring that ethical standards are met fosters public confidence in research practices and encourages greater participation, which is essential for scientific advancement. Safeguarding participants helps avoid historical pitfalls and promotes responsible collaboration across research institutions.
| Key Point | Explanation |
|---|---|
| Impact of Funding Cuts | Funding cuts, such as the $2 billion freeze in federal research grants by the Trump administration, disrupt essential oversight processes in medical research. |
| Role of Institutional Review Boards (IRBs) | IRBs are crucial for reviewing research proposals, ensuring participant safety, obtaining informed consent, and managing potential risks. |
| Need for Ethical Oversight | Ethical oversight is vital; past abuses in research led to the establishment of protective regulations to safeguard patient rights. |
| Collaborative Research Importance | SMART IRB facilitates collaboration among various research institutions, reducing bureaucratic delays in multi-center studies. |
| Risks of Halting Studies | Halting ongoing studies can endanger current participants and erode public trust in medical research as a whole. |
Summary
Patient safety in medical research is significantly impacted by funding cuts, which can disrupt vital oversight and ethical standards. The cessation of federal research grants can halt critical projects that protect the rights and well-being of clinical study participants. Institutional Review Boards (IRBs) play a fundamental role in ensuring that research is conducted ethically and safely, but without adequate funding and support, these systems can falter. Such disruptions not only endanger current research subjects but also damage public confidence in the integrity of medical research practices. It is crucial to recognize and advocate for the necessary funding to maintain patient safety in medical research to ensure that the health of communities is preserved.