TIM-3 therapy for Alzheimer’s is paving the way for innovative approaches in Alzheimer’s treatment by leveraging insights from the immune system. Recent research has identified TIM-3, an immune checkpoint molecule, as a significant player in the pathogenesis of Alzheimer’s, particularly in its late-onset form. By inhibiting TIM-3, scientists have demonstrated an impressive ability to revive the brain’s immune cells, known as microglia, which are essential for clearing harmful plaques and restoring cognitive function. This breakthrough offers hope for new therapies that may enhance memory and overall brain health in individuals afflicted by Alzheimer’s. With the potential of anti-TIM-3 antibodies to reshape Alzheimer’s treatment paradigms, the future of cognitive function improvement rests on harnessing the body’s own defenses against this devastating disease.
In the realm of Alzheimer’s disease management, TIM-3 inhibition emerges as a promising strategy to tackle the challenges posed by this neurodegenerative disorder. Utilizing immune checkpoint inhibitors, such as TIM-3, researchers aim to reactivate microglial activity, which has been suppressed as the disease progresses. By doing so, there’s potential to significantly diminish plaque accumulation in the brain, ultimately enhancing cognitive abilities. This innovative therapy not only offers a glimmer of hope for those suffering from Alzheimer’s but also expands our understanding of the potential link between immune regulation and memory safeguarding. As research continues to unfold, the application of anti-TIM-3 antibodies could revolutionize how we approach Alzheimer’s treatment, marking a crucial step forward in restoring brain function.
The Emergence of Immune Checkpoint Inhibitors in Alzheimer’s Treatment
In recent years, the application of immune checkpoint inhibitors, typically used in cancer therapies, has shown promise in the realm of Alzheimer’s treatment. These inhibitors, such as those targeting TIM-3, hold potential for transforming our understanding of neurodegenerative diseases. Notably, TIM-3 (T-cell immunoglobulin mucin-3), previously recognized for its role in preventing overactivation of T-cells in cancer, has been identified as a significant contributor to the pathophysiology of Alzheimer’s, especially in its late-onset form.
The association of TIM-3 with cognitive decline and plaque accumulation has spurred renewed interest in its therapeutic potential. As research indicates, deleting TIM-3 in animal models has led to enhanced microglial activity, enabling these immune cells to efficiently clear amyloid plaques from the brain, suggesting a pivotal role for TIM-3 therapy in improving cognitive function and combating Alzheimer’s.
Microglia’s Role and TIM-3 in Alzheimer’s Disease
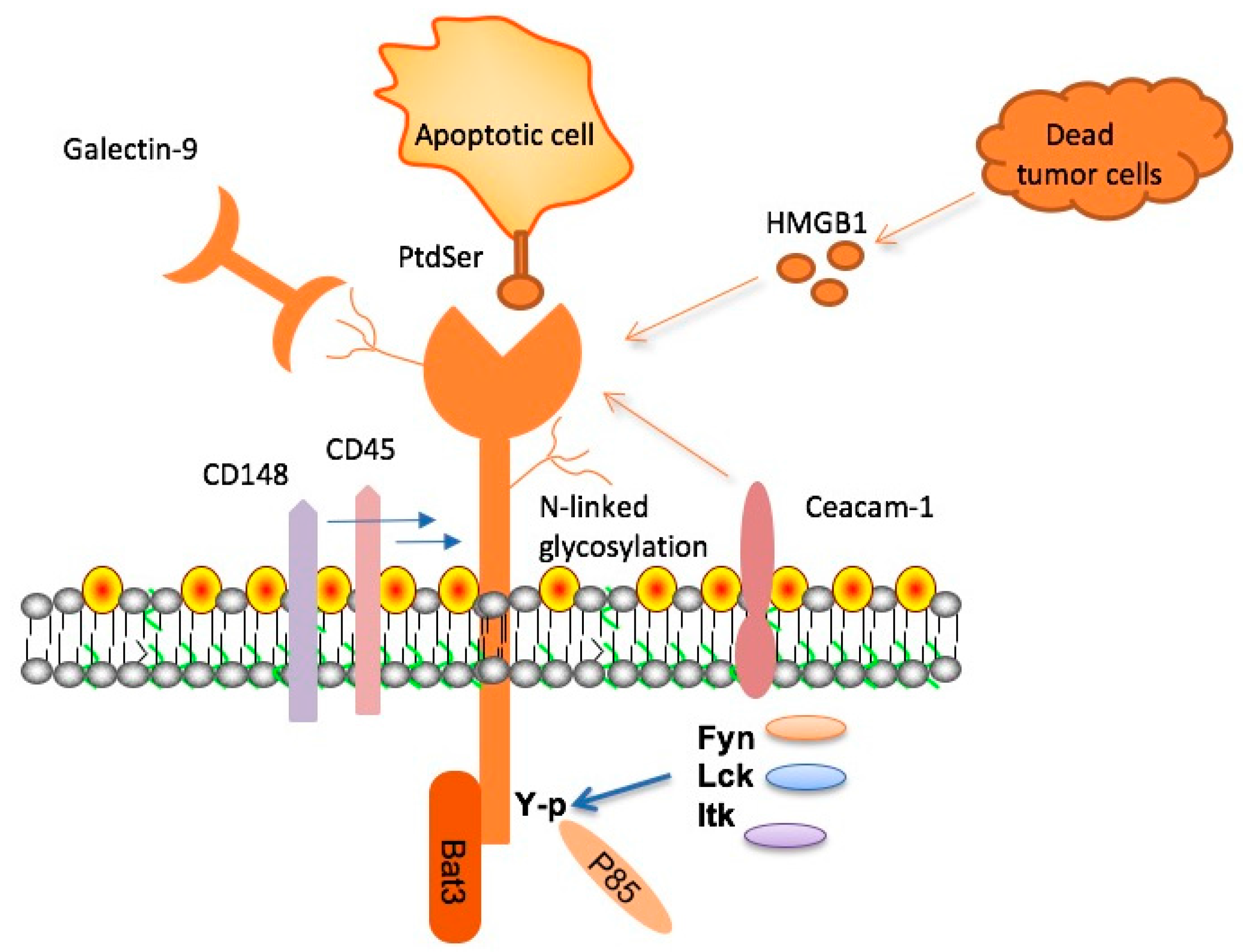
Microglia are the brain’s resident immune cells, crucial for maintaining homeostasis and clearing away damaged cells and debris. However, in Alzheimer’s disease, these microglia become dysfunctional, exacerbated by the expression of TIM-3, which inhibits their ability to attack harmful plaques. Elevated TIM-3 levels have been linked to the inability of microglia to perform their cleansing functions, leading to the accretion of amyloid-beta figures in the brain that are associated with significant cognitive impairment.
Addressing the challenge posed by TIM-3 could thus be central to innovative Alzheimer’s treatments. By inhibiting the TIM-3 pathway, we can potentially restore microglial function and enhance their ability to clear toxic plaque accumulations, thereby safeguarding cognitive function. The promise of TIM-3 therapy lies in its dual ability to rejuvenate microglial activity while also mitigating the neuroinflammatory processes that contribute to neuronal damage in Alzheimer’s.
Cognitive Function Improvement with Anti-TIM-3 Antibodies
Recent findings suggest that the administration of anti-TIM-3 antibodies may significantly enhance cognitive function in Alzheimer’s patients. Animal studies have demonstrated that these antibodies can effectively unleash microglia to remove amyloid plaques, leading to noticeable improvements in memory and learning capabilities. The potential for cognitive enhancement through the modulation of TIM-3 pathway represents a groundbreaking shift in Alzheimer’s research, offering hope for targeted therapies that could reverse some aspects of cognitive decline.
Clinical trials assessing the efficacy of anti-TIM-3 therapy are crucial for validating these findings in human subjects. If successful, this therapeutic avenue not only highlights TIM-3’s role as an immune checkpoint molecule but may also lead to new treatment protocols that prioritize immune modulation as a strategy in combating Alzheimer’s. Enhanced cognitive function as a result of such interventions may pave the way for broader applications of immunotherapy in neurodegenerative diseases.
Understanding Genetic Risk Factors in Alzheimer’s with TIM-3
Research has underscored the importance of genetic factors in the late-onset form of Alzheimer’s disease, with TIM-3 emerging as a notable risk marker. Individuals with certain polymorphisms in the HAVCR2 gene exhibit higher expression of TIM-3 in microglia, suggesting a genetic predisposition to the disease. This insight emphasizes the intricate relationship between immune checkpoint molecules and genetic risk factors associated with Alzheimer’s.
Understanding these genetic underpinnings not only aids in identifying at-risk populations but also opens avenues for personalized interventions, like TIM-3 targeted therapies. By focusing on individuals with specific genetic profiles, researchers can better tailor therapeutic strategies that may help mitigate the onset or progression of Alzheimer’s, pushing the boundaries of traditional treatment paradigms.
Mechanisms of TIM-3 in Neuroinflammation and Alzheimer’s
TIM-3’s role in regulating neuroinflammation represents a critical area of study in Alzheimer’s research. Neuroinflammation, largely driven by activated microglia, is a significant contributor to neuronal death and cognitive decline associated with Alzheimer’s. TIM-3 expression on activated microglia hinders their phagocytic capabilities, resulting in a detrimental environment that perpetuates plaque accumulation and neurotoxicity.
By elucidating how TIM-3 modulates the inflammatory response in the Alzheimer’s-affected brain, researchers are not only uncovering potential therapeutic targets but also offering insights into the biological processes underlying cognitive deterioration. This understanding is essential for developing interventions aimed at reestablishing homeostasis and curbing inflammation-driven neurodegeneration.
Potential of TIM-3 Targeting in Clinical Settings
The therapeutic potential of targeting TIM-3 is particularly significant given the historical failures of conventional Alzheimer’s treatments, which have often focused on amyloid-beta alone. By shifting towards a more comprehensive approach that includes immune modulation, such as TIM-3 therapy, we may unlock new pathways to alleviate the cognitive symptoms associated with Alzheimer’s disease. Utilizing existing anti-TIM-3 antibodies may accelerate the translational potential of novel interventions.
Exploring the use of TIM-3 inhibitors could herald a new chapter in the management of Alzheimer’s, facilitating the development of treatments that specifically enhance immune clearance mechanisms in the brain. As clinical trials continue to progress, the promise of TIM-3 targeting reflects a paradigm shift in our approach to Alzheimer’s therapy, focusing on the multifaceted nature of brain health and immune function.
The Future of Alzheimer’s Research with TIM-3
Research in Alzheimer’s is at a pivotal juncture, with the exploration of TIM-3 indicating a dynamic shift towards immunotherapy. By integrating knowledge from cancer therapy, scientists are uncovering possibilities to apply successful immune strategies to neurodegenerative conditions, illustrating the versatility of immune checkpoint molecules like TIM-3 in different disease contexts. The ongoing studies are not just transformative for treating Alzheimer’s but could redefine therapeutic strategies in chronic neurodegenerative diseases.
As we advance our understanding of TIM-3’s intricate role in Alzheimer’s pathology, clinical and preclinical trials will become vital in confirming its efficacy and safety in human applications. The potential integration of TIM-3 therapies into clinical practice could represent a significant breakthrough in not only improving cognitive functions but also in addressing the broader implications of neuroinflammation in aging populations.
Impact of Plaque Removal on Alzheimer’s Symptoms
Research has demonstrated a direct correlation between the clearance of amyloid plaques by microglia and the alleviation of Alzheimer’s symptoms. By utilizing strategies such as TIM-3 inhibition, the path to removing these plaques becomes clearer, potentially leading to improved cognitive outcomes for those affected by Alzheimer’s disease. This therapeutic avenue not only aims to restore cognitive function but also targets underlying cellular dysfunctions contributing to symptomatology.
As we delve deeper into the mechanisms of plaque formation and microglial function, the prospective outcomes of TIM-3-targeted therapies could change the landscape of Alzheimer’s treatment. The focus on plaque reduction underscores a critical shift towards treatments that prioritize the restoration of neural health, lending hope to millions affected by this debilitating condition.
Bridging Cancer and Alzheimer’s: Insights from TIM-3 Research
The crossover between cancer treatment methodologies and Alzheimer’s research is exemplified through the study of TIM-3. This molecule, primarily known for its role in cancer immunotherapy, offers unique insights as researchers apply similar concepts to neurodegenerative disease contexts. By understanding how TIM-3 modulation influences microglial action in both cancer and Alzheimer’s, we can potentially design synergistic therapies harnessing immune system capabilities.
This blending of disciplines not only enriches the scientific approach but also highlights the interconnectedness of immune responses across diseases. As discoveries in TIM-3 research evolve, they may lead to groundbreaking interventions that address both cognitive decline and tumor surveillance, further bridging the gap between cancer biology and neurobiology in therapeutic development.
Frequently Asked Questions
What is TIM-3 therapy for Alzheimer’s and how does it work?
TIM-3 therapy for Alzheimer’s involves the use of anti-TIM-3 antibodies to inhibit the TIM-3 molecule, allowing microglial cells in the brain to clear amyloid plaques that contribute to Alzheimer’s disease. By blocking TIM-3’s inhibitory function, these therapies aim to restore cognitive function by enabling microglia to attack and eliminate harmful plaques.
How does TIM-3 relate to immune checkpoint molecules in Alzheimer’s treatment?
TIM-3 is an immune checkpoint molecule that, when activated, inhibits the immune response in the brain. In the context of Alzheimer’s treatment, targeting TIM-3 can release this inhibition, empowering microglia to effectively clear amyloid plaques, thus potentially improving cognitive function in Alzheimer’s patients.
What is the significance of microglia and Alzheimer’s in relation to TIM-3 therapy?
Microglia are the brain’s immune cells responsible for clearing waste, including amyloid plaques associated with Alzheimer’s. TIM-3 therapy aims to enhance microglial activity by inhibiting the TIM-3 molecule, which currently prevents these cells from effectively performing their plaque-clearing function, ultimately aiming to promote cognitive function improvement.
Can TIM-3 therapy improve cognitive function in Alzheimer’s patients?
Yes, TIM-3 therapy has shown promise in preclinical studies, where the inhibition of TIM-3 allowed microglia to clear amyloid plaques, resulting in improved cognitive function in mouse models of Alzheimer’s disease. This suggests potential benefits for cognitive function improvement when applied to human patients.
What are the potential treatments using anti-TIM-3 antibodies for Alzheimer’s disease?
Anti-TIM-3 antibodies are being studied as a possible treatment for Alzheimer’s disease by blocking the TIM-3 molecule’s inhibitory effects on microglia. This therapeutic approach could lead to enhanced plaque clearance in the brain and subsequent improvement in cognitive function for Alzheimer’s patients.
What challenges exist in applying TIM-3 therapy for Alzheimer’s treatment in humans?
One challenge is ensuring that anti-TIM-3 antibodies can effectively reach the brain while avoiding the vascular issues seen with some anti-amyloid therapies. Ongoing research aims to optimize the delivery of TIM-3 inhibitors to maximize their effectiveness in treating Alzheimer’s patients.
What is the role of TIM-3 as a genetic risk factor in Alzheimer’s?
TIM-3 has been identified as a genetic risk factor for late-onset Alzheimer’s disease. A polymorphism in the TIM-3 gene may lead to impaired microglial function, which can contribute to the accumulation of amyloid plaques in the brain, thus playing a significant role in the progression of Alzheimer’s.
How long does the research and development of TIM-3 therapy for Alzheimer’s take?
Developing TIM-3 therapy for Alzheimer’s involves extensive research, typically spanning several years for each experimental phase. The work to understand TIM-3’s role in Alzheimer’s and explore therapeutic options has taken around five years, indicating the complexity of this scientific endeavor.
What future research directions are being pursued for TIM-3 therapy in Alzheimer’s?
Future research aims to test human anti-TIM-3 antibodies in mouse models of Alzheimer’s with humanized TIM-3 genes, evaluating their effectiveness in preventing plaque formation and improving cognitive outcomes in Alzheimer’s patients.
What have studies shown about the effects of deleting the TIM-3 gene in Alzheimer’s models?
Studies involving the deletion of the TIM-3 gene in mouse models have demonstrated enhanced clearance of amyloid plaques by microglia, leading to a reduction in plaque burden and some recovery of cognitive functions, highlighting the therapeutic potential of targeting TIM-3.
| Key Point | Details |
|---|---|
| TIM-3 and Alzheimer’s | TIM-3 is a checkpoint molecule linked to late-onset Alzheimer’s disease, inhibiting microglia from clearing amyloid plaques. |
| Research Findings | Deleting TIM-3 in mice allowed microglia to clear plaques more effectively and improved cognitive functions. |
| Mechanism of Action | Checkpoint molecules like TIM-3 prevent overactivity of immune cells; in Alzheimer’s, this hinders plaque clearance. |
| Potential Therapies | Therapies could involve anti-TIM-3 antibodies, which may enhance plaque clearance in humans. |
| Successful Approaches | Despite past failures in drug trials, the targeting of selective markers like TIM-3 presents a novel approach to treatment. |
Summary
TIM-3 therapy for Alzheimer’s is proving to be a promising avenue for treatment, as research reveals that inhibiting this checkpoint molecule can enable immune cells to effectively clear amyloid plaques from the brain. By unlocking the potential of microglia without the interference of TIM-3, cognitive function improvements have been observed in preclinical models. As researchers explore the use of anti-TIM-3 antibodies, this innovative strategy might pave the way for new clinical approaches that effectively combat Alzheimer’s disease.